|
Celebrating 35 years providing high quality products and advice.
|
| Our Local Time Is 7:25:28 AM. |
| Call us at 818-786-0600. We are here to help! |
Mercury Contamination
The effluents of many industrial processes, as well as surface water and groundwater from historically polluted sites, often contain unacceptably high levels of Mercury and other toxic trace metals. Although inorganic Hg itself is not bioaccumulative, it is readily converted to methyl mercury in the ambient environment, and so should be removed before discharge. The U.S. EPA has identified mercury-contaminated groundwater and surface water as areas of particular concern, and has emphasized development of innovative, robust extraction technologies.
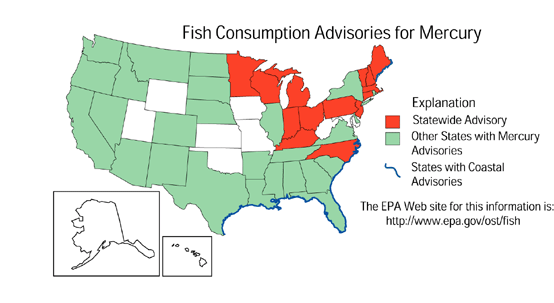
Mercury contamination is a significant problem for aquatic organisms and predators as well as a potential health risk for humans. Many lakes in the northern United States, southern Canada, and Scandinavia are affected by mercury contamination (Mierle, 1990; Hurley and others, 1991; Lindqvist, 1991). Florida and 27 other states have issued health advisories that restrict consumption of fish because of high levels of mercury (Tom Atkeson, Florida Department of Environmental Regulation, written commun., 1992). The problems with mercury relate to two factors--mercury biomagnifies in the food chain to toxic concentrations, even though it is found at very low (subnanogram-per-liter) concentrations in water; and mercury is transported and deposited through atmospheric processes and is, thus, widely distributed. Methylmercury is the more toxic bioaccumulative species. As a result of environmental changes, increased rates of methylation may be contributing to toxic mercury problems. For example, a change from an aerobic to an anaerobic environment would favor increased mercury methylation (Winfrey and Rudd, 1990; Gilmour and Henry, 1991) and mercury uptake in the biota. Because of the existing burden of mercury in the environment, recycling of mercury between demethylated and methylated forms can provide a source of methylmercury even if atmospheric deposition ceased. However, estimates indicate that atmospheric mercury concentrations have nearly doubled over the last 50 years (Fitzgerald and others, 1991; Slemr and Langer, 1992). Increasingly evident is the fact that even modest increases in atmospheric deposition can translate into mercury levels in organisms that are of ecological and, perhaps, toxicological concern.
Remove Mercury with :
Mercury Removal Filters
Reverse Osmosis
Distillation Systems
|
|

