|
Celebrating 33 years providing high quality products and advice.
|
| Our Local Time Is 1:18:33 AM. |
| Call us at 818-786-0600. We are here to help! |
 |
ON SALE NOW
Introducing the Polaris Lab Water Systems
High Purity Water Made In The USA.
Click here for more info. |
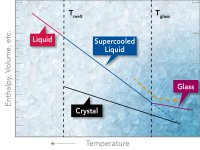
What is Supercooled Water
|
Using additives can lower the freezing temperature of water. For example sprinkling salt on ice causes the ice to melt. Antifreeze solutions added are added to car radiators to protect them from sub zero temperatures. However lowering the freezing point in this manner is not supercooling.
It should be pointed out that even without additives water may not solidify when the temperature is 0oC or slightly below if the water is pure and not being agitated. What occurs is a standoff between the cyrstallizing forces of the molecular alignment based on hydrogen bonds and the decreasing volume that accompanies reduced molecular movement. This balance is very delicate. A speck of dust or a minor vibration can cause crystallization. Until this happens the water temperature can drop a degree or more below 0oC without crystallization. When water is in this condition it is said to be supercooled.
More Information About Water
Molecular Structure
The Three States
Sublimation
Supercooled Water
Heavy Water
Properties
Heat Capacity
Latent Heats
Solvent
Surface Tension
| |
|
Images are representative of the products. Images may or may not be of the actual product. If it is important e-mail us for an actual image if available.
* Flat Rate UPS shipping when able to ship via UPS and is in the USA excluding Hawaii and Alaska.
Larger Items may not be able to ship via UPS, in that case freight charges will be quoted seperately.
International shipping will be quoted after the order is placed. You will have the opportunity to cancel before we finalize your order.
Terms and conditions
Credit Application
Privacy
Policy
List All Products
|